Protection against bacteria, viruses and fungi
with graphene coatings
In less than 20 years the world has faced a series of abnormal phenomena caused by highly infectious pathogens. The easy and rapid transmission of infections forces us to seek increasingly efficient strategies to strengthen health services, in addition to representing a radical change in our lifestyle, where extreme hygiene techniques are in first place of importance to avoid the spread and massive contagion inside and outside hospitals.
Viral diseases of greater impact.
- 2002-2003. Severe acute respiratory syndrome (SARS-Cov).
- 2012. Middle East Respiratory Syndrome (MERS-Cov).
- 2014- 2016. Ebola.
- 2019- 2022. SARS-Cov-2.
>6.5 million deaths.
Dangerous bacteria for human health:
- Staphylococcus aureus.
- Streptococcus pneumoniae.
- Pseudomonas aeruginosa.
- Haemophilus influenzae.
- Helicobacter pylori.
Common fungi in the domestic environment:
- Aspergillus spp.
- Cladosporium spp.
- Alternaria spp.
- Acremonium spp.
- Epiccocum spp.
- Penicillium spp.
- Stachybotrys spp.
Graphene as an adjuvant in infection control
In 2018, Energeia- Graphenemex® launched the antimicrobial Graphenergy line, made up of two specialized vinyl- and vinyl-acrylic-based coatings with graphene oxide, whose antimicrobial potential is 400 times higher than common products, helping to keep surfaces free of fungi and bacteria for a long time.
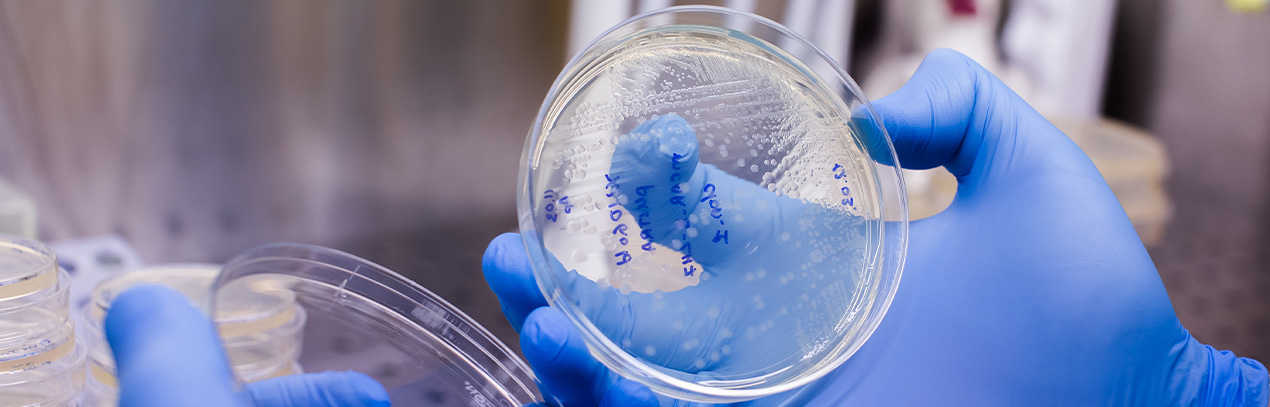
In vitro studies and in a relevant environment carried out by the Laboratory of Pathology, Biochemistry and Microbiology of the Faculty of Stomatology of the U.A.S.L.P., showed that surfaces protected with antimicrobial Graphenergy remain free of microorganisms for more than 6 months, without the need for additional chemicals. Figure 1.
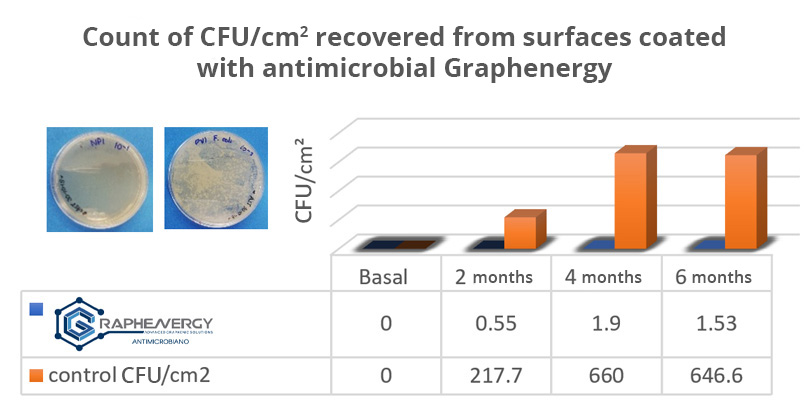
Important: A clean surface is in the range of 1-10 CFU/cm2.
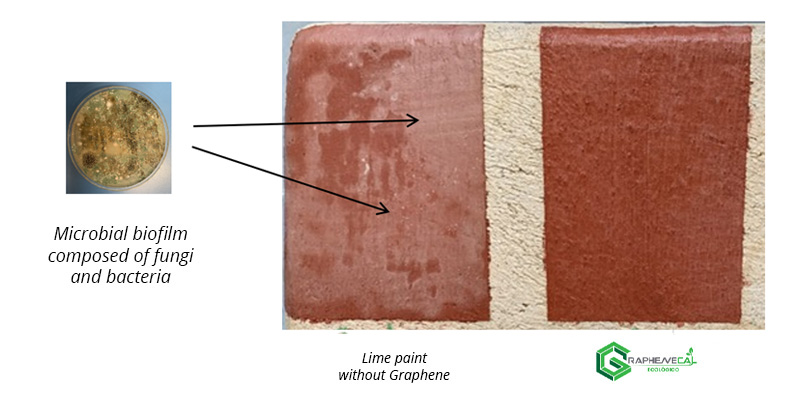
In 2022, the strategic alliance between the companies Energeia-Graphenemex® and Oxical® is preparing to launch a new 100% natural coating, without toxic compounds (VOCs), highly waterproof, breathable and highly antimicrobial, made from high-quality and purity lime modified with Graphene nanoparticles, under the ecological Graphenecal brand.
Its extraordinary antimicrobial capacity is not only a great aid in keeping spaces free of microorganisms, but also protects surfaces against biodeterioration, particularly those with high historical value. Figure 2.
Is graphene nanotechnology safe?
Yes, Graphenergy and Graphenecal antimicrobial coatings are as safe as any conventional paint or coating. The graphene and graphene oxide nanoparticles contained in its formulations do not shed or release toxic substances into the environment.
“Not all microorganisms are dangerous, but it is better to keep them away”
How do graphene materials work?
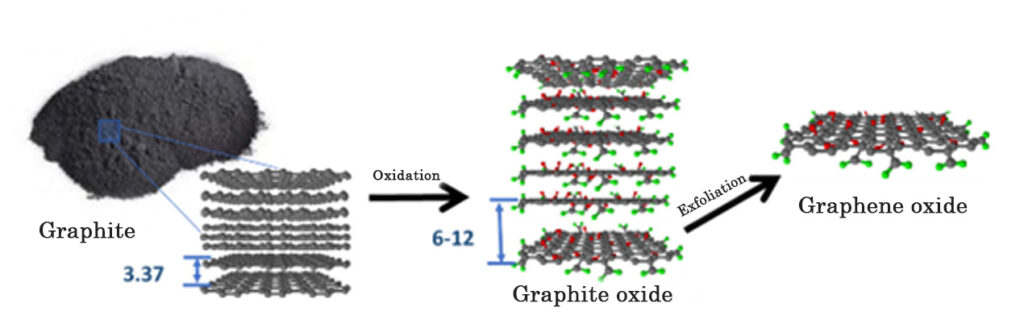
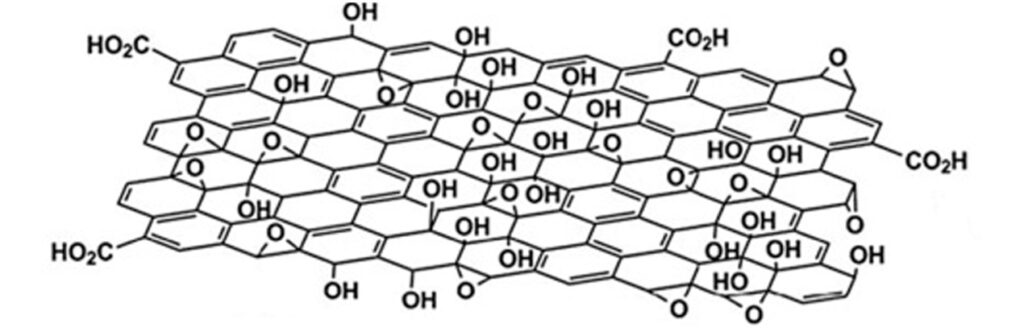
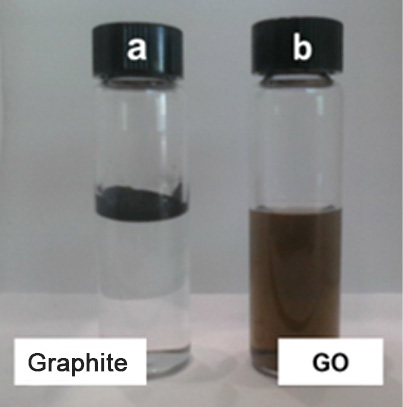
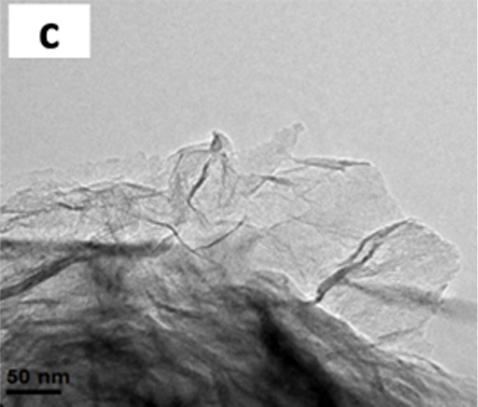
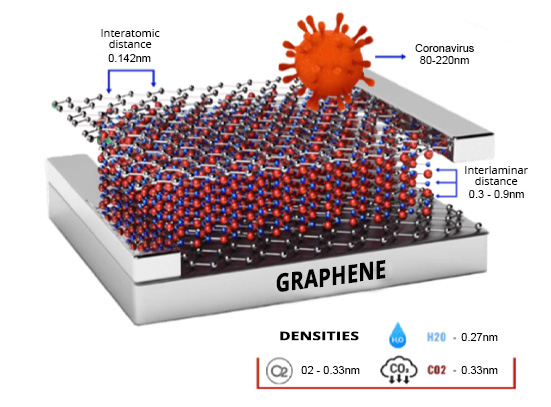
- Physical barrier- High impermeability. Graphene materials are usually presented in millions of blocks composed of 1 to 10 nanometric sheets similar to a pack of cards, with multiple sinuous paths between each sheet that act as an external barrier that suppresses the entry of essential nutrients for microbial growth.
- Graphene and its derivatives can act as electron donors or acceptors, altering the respiratory chain of the microorganism or extracting its electrons. This imbalance in the form of a nano-circuit is so fast that it does not give the microorganism time to recover and, therefore, inactivates it before adhering to the surface.
- Structural damage. The edges of the nanomaterial sheets act like small knives that damage or break the cell membrane of the microorganism, altering its functioning and preventing its viability.
Do graphene materials have antiviral activity?
The antiviral effect of graphene materials seems not to be very different from that described against fungi and bacteria. The hypotheses are directed towards an interesting synergistic effect between impermeability, structural damage and electrostatic interactions due to the positive polarity of some viruses (SARS-Cov-2) and the negative polarity of graphene oxide, in addition to its great protein-anchoring capacity.
Energeia- Graphenemex®is the pioneer Mexican company in Latin America focused on the research and production of graphene materials for the development of applications at an industrial level. In addition to adding value to its products with the multifunctional properties of Graphene and its derivatives, the company also aims to create strategic alliances to support innovative developments with graphene nanotechnology.
References
- García-Contreras R, Guzmán Juárez H, López-Ramos D & Alvarez Gayosso C. Biological and physico-mechanical properties of poly (methyl methacrylate) enriched with graphene oxide as a potential biomaterial. J Oral Res 2021; 10(2):1-9. Doi:10.17126/joralres. 2021.019
- UM.D. Giulio, R. Zappacosta, S.D. Lodovico, E.D. Campli, G. Siani, A. Fontana, L. Cellini, Antimicrobial and antibiofilm eficacy of graphene oxide against chronic wound microorganisms. Antimicrob. Agents Chemother. 62(7), e00547-18 (2018). https://doi.org/10.1128/AAC.00547-18
- H.E. Karahan, C. Wiraja, C. Xu, J. Wei, Y. Wang, L. Wang, F. Liu, Y. Chen, Graphene materials in antimicrobial nanomedicine: current status and future perspectives. Adv. Healthc. Mater. 7(13), 1701406 (2018). https://doi.org/10.1002/ adhm.201701406
- Sydlik SA, Jhunjhunwala S, Webber MJ, Anderson DG, Langer R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano. 2015. 9: 3866
- Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010. 10: 3318.
- Bhattacharya K, Farcal LR, Fadeel B. Shifting identities of metal oxide nanoparticles: focus on inflammation. 2014. MRS Bull; 39: 970
- Huang PJ, Pautler R, Shanmugaraj J, Labbé G, Liu J. Inhibiting the VIM-2 metallo-β-lactamase by graphene oxide and carbon nanotubes. ACS Appl Mater Interfaces 2015; 7: 9898.
- Moghimi SM, Wibroe PP, Wu L, Farhangrazi ZS. Insidious pathogen-mimicking properties of nanoparticles in triggering the lectin pathway of the complement system. Eur J Nanomedicine. 2015; 7: 263.
- Bhattacharya K, Mukherjee SP., Gallud A., Burkert SC., Bistarelli S., Bellucci S., Bottini, M., Star A., Fadeel B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine: Nanotechnology, Biology, and Medicine. 2016. 12. 333