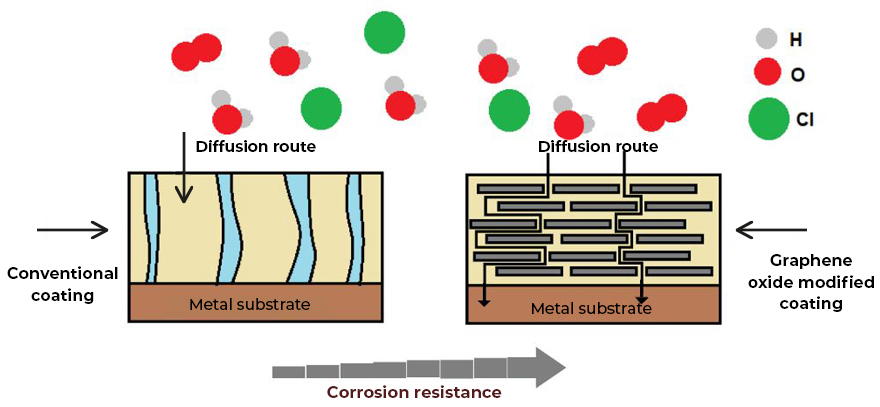
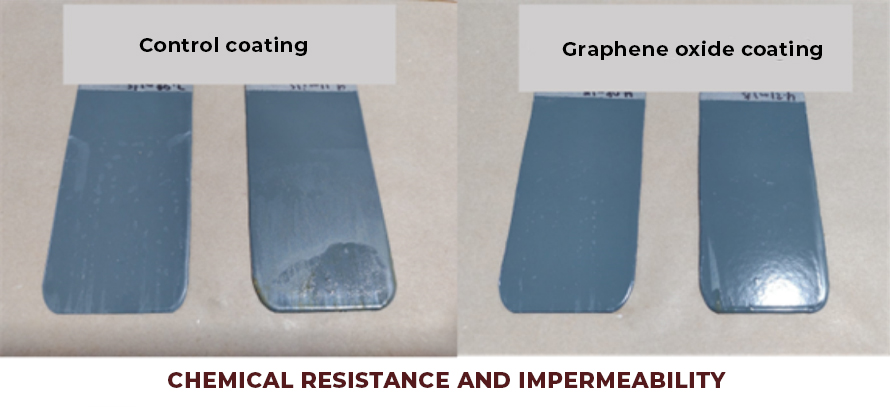
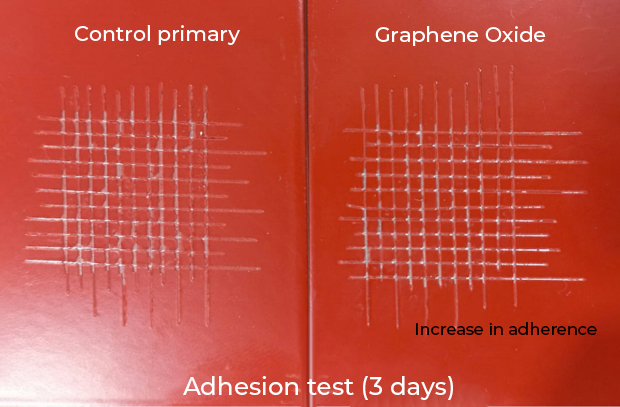
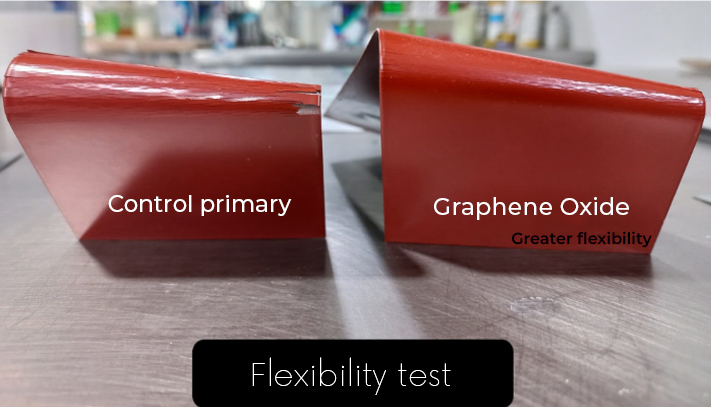
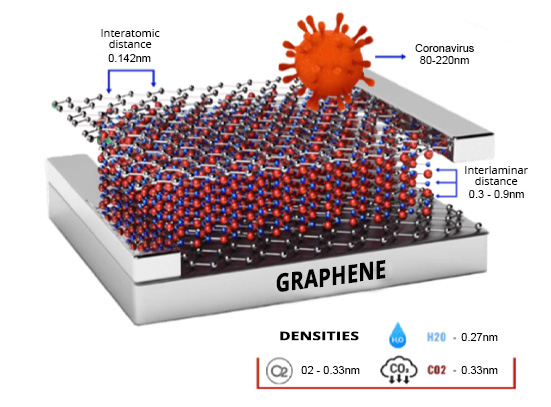
Graphene:
The next revolution in biomedical applications
Part I. Tissue Engineering
Advances in medicine have reached levels unimagined until recently. Among them, tissue engineering has an important participation. With it is possible to combine cells, biomaterials and biologically active molecules with the aim of repairing or replicating tissues or organs with a function similar to that of the original structure. In principle, biomaterials are used as molecular scaffolds to act as a three-dimensional (3D) support or guide for the anchoring and growth of the cells that will be in charge of forming the new tissue.
The first molecular scaffolds were designed with natural materials such as collagen, glycosaminoglycans (GAGs), chitosan, and alginates; then with artificial compounds such as polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic) acid (PLGA), polyurethanes (PUs), polytetrafluoroethylene (PTFE), polyethyleneterephthalate (PET); bioceramics such as hydroxyapatite (HA) and tricalcium phosphate; metals such as stainless steel, chrome-cobalt alloys (Co-Cr) or titanium alloys (Ti) and recently, new research is oriented towards the use of nanotechnology.
The relationship between nanotechnology and tissue engineering is due to the fact, that the extracellular matrix (ECM) that helps cells unite and communicate with each other, is made up of a network of nanometer-sized fibers made up of bioactive molecules. It is at this point where nanotechnology opens new possibilities for regenerative medicine, since it has been proven that the use of materials that act on the same nanometric scale as the ECM favors mimicking the physiological environment of the organism to stimulate cell growth and differentiation in a more natural environment.
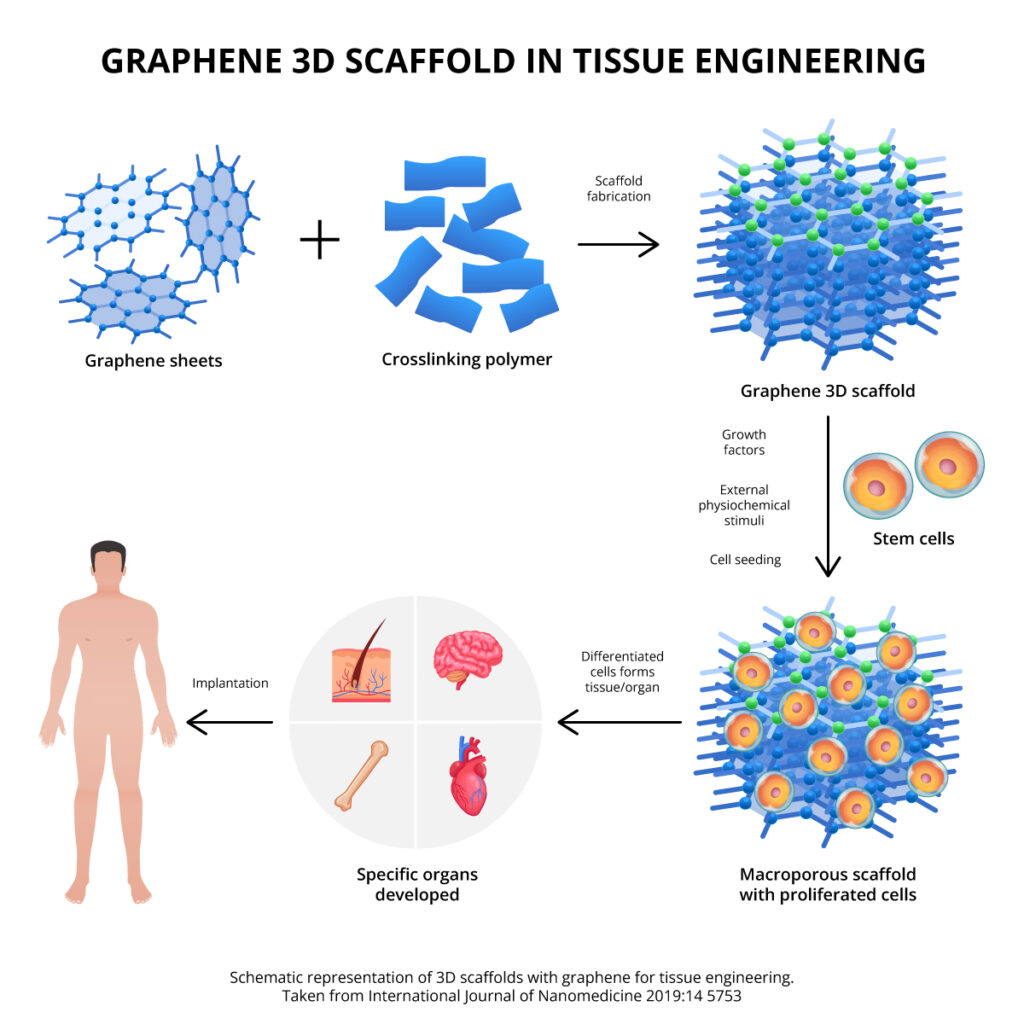
Among the most studied nanomaterials in recent years are graphene materials, which consist of nanometric sheets of carbon atoms organized in two-dimensional (2D) hexagonal networks. Among the most interesting properties for tissue engineering are: its large surface area, mechanical resistance, thermal conductivity, biocompatibility and finally, an extraordinary ability to share its properties with other materials to improve their original characteristics.
For example, the use of graphene materials within the 3D architecture of certain biopolymers in tests carried out on heart, liver, bone, cartilage, and skin tissues has shown substantial improvements in their physicochemical, mechanical, electrical and biological properties, achieving excellent response. for stem cell adhesion and differentiation.
In 2022, the Andaltec technology center (Spain) reported the development of a material from polymers derived from graphene by 3D printing with great potential for the regeneration of muscle tissue. They demonstrated that in the presence of graphene derivatives, cells contract and expand without an external stimulus, therefore, it has great potential for use in regenerative medicine.
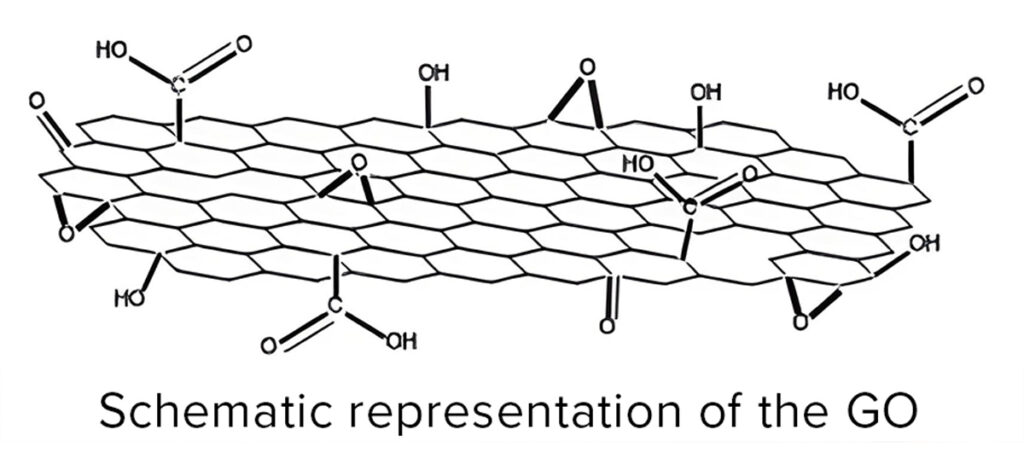
On the other hand, the Division of Postgraduate Studies and Research (DEPeI) on Odontology, UNAM and the National School of Higher Studies (ENES) León Unit, Mx., through a study published in J Oral Res 2021 supports the possibilities of graphene oxide (GO) in the design of biomaterials for dental use. The results of the research carried out with Graphenemex® GO samples, concluded that this nanomaterial in combination with polymethylmethacrylate (PMMA), in addition to improving its physical-mechanical properties, also demonstrated good compatibility and an interesting stimulation of cell proliferation when being evaluated on cultures with gingival-fibroblasts, dental-pulp-cells and human osteoblasts.
In 2020, researchers from the University of Malaga (Spain) published another study that identified GO as the ideal material for regenerative medicine. The study carried out on an animal model, showed high biocompatibility of different types of graphene oxide with dopaminergic cells, favoring their maturation and protecting them from the toxic conditions of Parkinson’s disease. These results postulate GO as an adequate scaffold to test new drugs or develop constructs for cell replacement therapy of Parkinson’s disease.
Despite the large amount of research on the interactions of graphene materials with biological media, there is still a long way to go to have these biomaterials available and in clinical operation. Energeia- Graphenemex, the pioneering Mexican company in Latin America in the research and development of applications with graphene materials, in collaboration with other companies and research centers, seeks to contribute with science to understand these interactions in a security framework, to lay solid foundations on the use of graphene nanotechnology in the biomedical sector for the benefit of society.
Drafting: EF/DHS
References
- Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications. J Nanostructure Chem, 2022, 12:693
- Biological and physico-mechanical properties of poly (methyl methacrylate) enriched with graphene oxide as a potential biomaterial. J Oral Res 2021; 10(2):1
- Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2021, 22, 250
- Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int J Nanomed 2020:15 2501
- Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci., 21 December 2020. Sec. Neuropharmacology Volume 14
- International Journal of Nanomedicine 2019:14 5753
- Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mat. Interfaces 2019, 6, 1900229
- Graphene based scaffolds on bone tissue engineering. Bioengineered, 2018, 9:1, 38
- When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials, 2018, 155, 236
- Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016,105, 255
Chapter 92 e: Tissue Engineering, Anthony Atala. 2023 McGraw Hill.